Updated June 14, 2023
If you have a small dog, there’s a good chance they’ll end up being treated with pimobendan. Often sold as Vetmedin® or Cardisure®, it’s the most common treatment for a very common condition: heart disease in dogs.
One disease in particular, myxomatous mitral valve disease (MMVD) is said to account for 75% of heart disease and affects 85% of small dogs over 13 years old. Not all of these will need treatment, but many will. Later I’ll show you how to tell.
There is no question that recent advances have made MMVD much less of a death sentence than it once was. So it’s good to get to know pimobendan, the newest, and most commonly used of these treatments.
How Pimobendan Works
Pimobendan is a drug that unusually has two separate positive effects. First, it increases the strength of contraction of the heart, increasing the amount of blood delivered. Second, it dilates blood vessels in the general circulation, reducing the heart’s workload.
Pimobendan needs to be given at a dose of 0.2–0.3 mg/kg twice a day on an empty stomach, leading to the following inescapable logic. If it needs to be given one hour before any food and 12 hours apart, then even starting with a 7am dose will result in an 8pm dinner time. Now of course, we don’t recommend feeding dogs after dark…
You can picture the disappointment in peoples’ faces when they think this through. It’s definitely a drug for the early risers in the house. The only other alternative is to make their main meal in the morning.
Lifespan Of Dogs On Pimobendan
Early work demonstrated that pimobendan could help dogs with MMVD, but by how much was uncertain, and so vets like me were slow to change treatments that were already working. Then two large international studies appeared. With them came a sea-change in how we viewed this drug.
Each of them has something important to say about both effects and side effects. Both are referenced below.
2008: The QUEST Study
252 dogs with naturally occurring MMVD and congestive heart failure were divided into two groups: one taking pimobendan and another taking benazepril, the leading heart treatment at the time. Both were allowed other treatments as needed. They were then studied over the following years until one of the following three things happened:
- sudden death
- euthanasia for cardiac reasons
- treatment failure
For pimobendan, the median time to this endpoint was 188 days. For benazepril it was 140. So a good result, but far from impressive. However, it’s worth pointing out here that these survival times are artificially short; many dogs in the study had already been affected for some time before beginning.
Real life is better. The point is more that in matched groups, pimobendan outperformed its rival.
2016: The EPIC Study
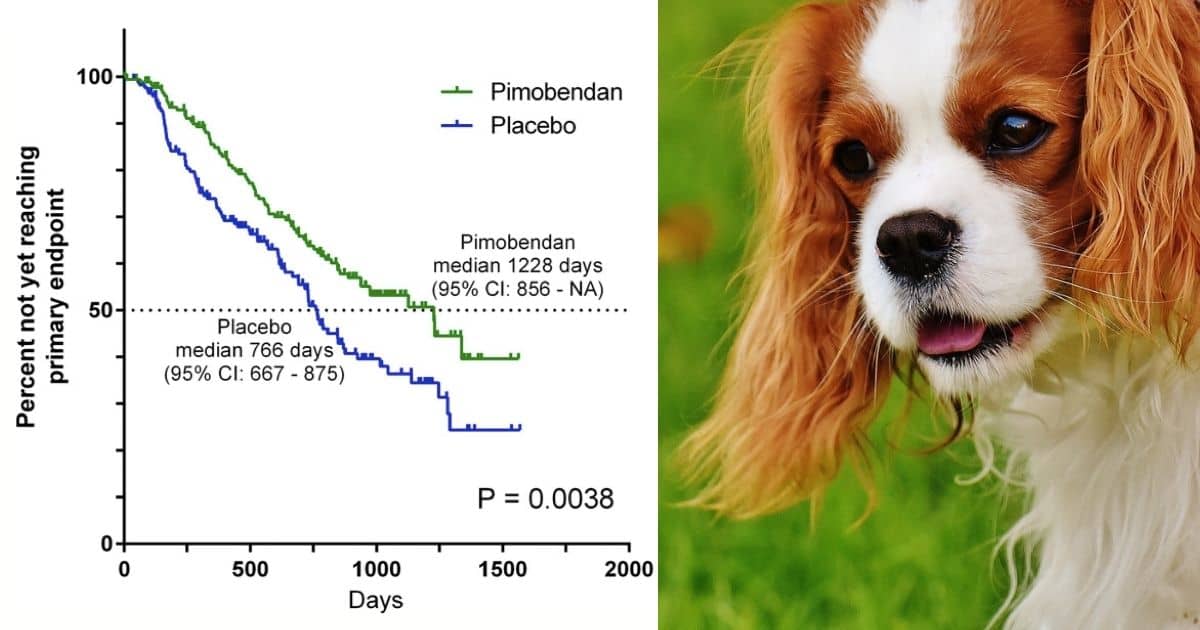
To my knowledge, the EPIC study was the first in veterinary medicine to be stopped early because of what was found. 354 dogs were chosen who had MMVD but were not yet in heart failure. At this earlier stage they had enlarged hearts as determined by xray and ultrasound.
Up to the time of the study, treatment was not believed to help at this stage, and so they were divided into dogs given pimobendan and dogs given a harmless placebo instead. This time the endpoint was chosen to be one of:
- development of left-sided heart failure
- euthanasia for a cardiac reason
- death presumed to be cardiac in origin
The median time to this endpoint was 1228 days in the pimobendan group and 766 days in the placebo group. In other words, dogs with enlarged hearts but without heart failure had an extra 60% or 462 days of disease-free life if they took pimobendan.
This of course was a stunning result. Once it became obvious, all of the dogs were put on pimobendan.
Pimobendan Side Effects
Similar rates of adverse effects were reported for pimobendan and benazepril. This suggests that the drug is at least as safe as other heart treatments,
Pimobendan also recorded similar side effects to the placebo. Deaths in the pimobendan group were 46.4% versus 57.2% in the placebo group. This suggests that pimobendan is safe compared with any drug.
If you want to take a closer look, I’ve included the reported side effects from both studies in two tables after the references.
Help! Pimobendan Killed My Dog!
What then do we make of online reports of terrible events after dogs took pimobendan? The high rates of death and side effects in the placebo group provide the best clue. These are old dogs with a high risk of illness from any cause.
We humans are notoriously bad at separating causation from correlation. In fact, with any one dog, it’s virtually impossible to decide if a sudden death is caused by a drug or not. It’s only by looking at large groups that we can see the trend. Sometimes it’s real, other times it’s not.
Pimobendan may in fact cause deaths in some dogs. Even if so, the evidence tells us that they are heavily outweighed by the dogs that survive for longer.
When Should My Dog Take Pimobendan?
Pimobendan is a much better drug at stopping dogs going into heart failure than it is in treating them once they do. So here’s a quick summary of how to use pimobendan in 2022 and beyond:
- Get regular checkups (at least annually) to look for early signs of heart disease. This is mainly the appearance of a heart murmur.
- Once a murmur appears, watch fitness, coughing and resting respiratory rate closely and get a checkup at least every 6 months.
- Follow your vet’s advice on further testing. Sooner rather than later they will want to do chest xrays and possibly cardiac ultrasound to look for the signs of heart enlargement.
- Even if things are normal, expect things to change and so repeat the tests every 6 to 12 months based on your vet’s advice. Eventually you’re likely to spot the right time to start pimobendan.
- Once started, most heart disease will stabilise but dose adjustments and extra medications will still be necessary as the disease slowly worsens (hopefully over years, not months). Therefore, keep attending scheduled checkups and get advice straight away if anything changes.
Most importantly, trust the science. It’s very hard to judge the efficacy of any treatment used to prevent a disease instead of treat it, but we actually have a lot to go on here.
Related: A Dog With Dilated Cardiomyopathy (also treated with pimobendan) caused by a grain-free diet
Update: Mitral Valve Repair
I hesitate to add this as I fear giving dog owners false hope, but several comments below have spurred me to also discuss the surgical option. As of 2023, there are now several centres around the world that offer open heart surgery to improve mitral valve function. It is not replacement of the valve, but instead modifying its shape or adding support so that the leak is less significant.
Costs are extreme (I would guess $40-50K) and availability currently limited to the UK, France, Japan and the USA. If you Google ‘dog mitral valve repair’ you should find useful information from these sites. There is no doubt that this procedure will become more commonplace with time, and hopefully more realistic.
Have something to add? Comments (if open) will appear within 24 hours.
By Andrew Spanner BVSc(Hons) MVetStud, a vet in Adelaide, Australia. Meet his team here.
References
Boswood, A., Häggström, J., Gordon, S. G., Wess, G., Stepien, R. L., Oyama, M. A., … & Watson, P. (2016). Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC study—a randomized clinical trial. Journal of Veterinary Internal Medicine 30,(6), 1765-1779. Full Article.
Häggström, J., Boswood, A., O’grady, M., Jöns, O., Smith, S., Swift, S., … & DiFruscia, R. (2008). Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. Journal of Veterinary Internal Medicine, 22(5), 1124-1135. Full Article.
Keene, B. W., Atkins, C. E., Bonagura, J. D., Fox, P. R., Häggström, J., Fuentes, V. L., … & Uechi, M. (2019). ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. Journal of veterinary internal medicine, 33(3), 1127-1140. Full Article.
| Observed Adverse Events | Pimobendan (124) | Benazepril (128) |
| Gastrointestinal disorders (eg, vomiting, diarrhea, anorexia) | 6 | 4 |
| Abnormal behavior (eg, lethargy,confusion, uneasiness) | 3 | 4 |
| Tachycardia (supra or ventricular or both) | 1 | 1 |
| Seizure | 3 | — |
| Polyuria, polydipsia, incontinence | 1 | 2 |
| Dyspnea (intermittent) | 1 | 2 |
| Hepatic enzyme elevation | 2 | — |
| Syncope | 1 | 1 |
| Keratoconjunctivitis | — | 1 |
| Otitis externa | — | 1 |
| Purulent local dermatitis | — | 1 |
| Total | 18 | 17 |
| Pimobendan N = 179 | Placebo N = 180 | |
| Number of dogs experiencing at least 1 severe or worse adverse event | 19 (10.6%) | 19 (10.6%) |
| Number of dogs experiencing at least 1 mild or moderate adverse event (but not a severe or worse event) | 61 (34.1%) | 67 (37.2%) |
| Number of dogs experiencing no adverse events | 99 (55.3%) | 94 (52.2%) |
| Number of recorded adverse events | ||
| Severe or worse | 23 | 21 |
| Mild or moderate | 145 | 153 |
| Total | 168 | 174 |
| Frequency of specifically recorded adverse events | ||
| Diarrhea | 21 | 14 |
| Vomiting | 27 | 27 |
| Anorexia | 7 | 12 |
| Lethargy | 13 | 15 |
| Tachycardia | 4 | 3 |
| Other | 124 | 147 |
| Total | 196 | 218 |

My Chauauaua was DIOGNIOSED with heart failure put on perscribed meds but the side effects became violent. I weaned off against my vets advice because quality of life should be considered she immediately started coughing but not all the time. She just had a bad bout with Colin’s . Distorted her on Antibiotics . She is still having s hard time pooping. Her breathing is labored now to the extent of her comming to me for help. Please perscribe a breathing med that I can give this sweet 16 year old that will not make her sick. She suffered enough.
Hi Olivia. Most of these dogs will improve their breathing with the use of a diuretic like frusemide, but it has to be used carefully to avoid kidney damage. It will probably shorten overall lifespan, but improve quality of life in the meantime. You can also ask your vet about using an ACE inhibitor, instead of pimobendan, if it makes your dog sick.
I have a 14 yr old chihuahua, always been healthy until late October. She had a cord break suddenly in her heart, she became weak & peed on the floor. We took the necessary steps to get her ER care, then cardiovascular after, followed by regular visits with her general vet. She was doing very well being on pimobendan, furosemide, Enalapril & spironolactone. 4 weeks ago, we took her to a new cardiologist because our other retired. They increased her furosemide to 3x daily and she began passing out while pooping. The cardiologist increased her pimobendan to 3x per day and she stopped losing consciousness but was having daily weak spells usually while peeing. They increase her furosemide again to an higher dose and now she has labored breathing constantly. Is this the normal progression of CHF or is she on too much medication? It’s so odd that right after her increase in medication, like the week after, she began to decline in health. Am I just in denial here? Thank you so much!
Hi LeAnn. Unfortunately, this sounds like the normal progression of CHF. Increasing the frusemide dose should not cause a dog to pass out or have dizzy spells, instead, its side effects would normally be problems with hydration or kidney function. In fact, the frusemide is usually being used to improve laboured breathing. What they appear to be doing is trying to chase the worsening of the cardiac disease with higher and more frequent doses (I would probably have done the same), but it doesn’t sound like there is too much more that can be done.
I run a nonprofit rescue and was blessed to rescue a puppy mill mini schnauzer who has had several medical issues so we didn’t offer her for adoption. I worked with her to help her socialize. She had serious kidney issues and we were fortunate enough to help cure her with a good diet and consistent environment. She is now 10 years old and has a very enlarged heart. Never coughed but it showed on X-ray. Her heart fills 90% of her chest. She is on pimobendan twice daily and does pretty well but her appetite is not good now. Any suggestions on how I might help her? She had such a terrible life and had just began to enjoy herself. I love her so much and will do anything to help her. I changed her name to MinnieMe because she does everything possible to be by my side.
Hi Anita. Unless she is in late stage, cardiac disease, the appetite loss is unlikely to be caused by the heart. I’m afraid there may be another problem that requires investigation.
Nobody seems aware of MVD repair that’s available in England and Japan . It’s expensive but an amazing breakthrough and worth everyone exploring. It saved my babies life x
Hi Suzanne. It’s a very promising technique, but as yet out of reach of most dog owners for location and price reasons. We’re certainly watching the area closely.
Hi Suzanne, did you have your dogs MVD repair done overseas and may I ask was it expensive. I own a maltase she is 15 years old with this problem and all the vet seems to say not much can be done just increase the meds. I am so disappointed they don’t fix the cause of the problem just treat the symptoms. I just don’t know how much more time we have and I’m sure its quite a bit of organizing to get this done. Could you advise me on what’s involved.
Regards Tony
Hi Tony. I’ve added a small comment at the bottom of the article, from which you should be able to find the various centres that perform the operation.
Hi My 13 year old Cavoodle has just been put on Cardisure due to leaky valve and murmur. I understand that it should be taken an hour before meals but could I give her the tablets after meals instead. If so, would I need to allow 4 hours after for an empty stomach? She has also been prescribed Sildenafil for high blood pressure in the heart. Does Cardisure alone not help with that? Thank you also for the information on ‘grain free diets’ I thought I was giving my dog a healthier diet but will swap back after reading your article.
Hi Karen. I would advise against giving pimobendan after meals unless you have good data on stomach emptying times for your chosen food. I know it’s very hard to give on an empty stomach, but the flavoured tablets certainly help (though not with the early start!) If you need to use food, try wrapping in shaved ham, or something else very minimal so that you don’t interfere with absorption. Regarding sildenafil, no, it won’t have the same effect at all.
For medicines I used a gelatin cap from the vitamin shop, put the pill in it and then used “Wellness canned dog food” rolled a small amount and then put the pimopedan in it. I used the limited ingredient turkey and potatoe. My dog is allergic to Chicken and Wellness is low in sodium compared to other dog foods (canned). I used it to give him medicines. My Dog had CHF was on pimobedan, furesimide and entresto for 2 years. We reduced the furesimide amount the 2nd week of treatment. My dog was fortunate and we had him operated on in England at a placed called DWR. It has been 3 months since the surgery and he is doing well. His heart is now normal and no murmur. Our last dog developed this at 16.5 years old so I have had experience with it.
I took my 12 yo Pomeranian to the Vet, they put him on 1.25 Vetmedin twice a day & one Anapril a day. I wanted a more in depth look so I took him to a cardiologist. The cardiologist said, my Pom has a heart murmur 4 out of 6 but his heart enlargement is just in the beginning stage. He prescribed compounded 1.25 Pimobendan twice a day and said no need for Anapril yet. What are your thoughts? Thank you
I agree completely!
Our cavalier was diagnosed with stage 3 MVD last summer. He had no noticeable symptoms but the murmur was discovered during a routine exam. He was put on Vetmedin at that time. Since beginning the medication he has had increased polyuria to the point that he once only needed to go out three times a day (we live in a condo) to now sometimes he’ll have an accident after less than two hours. There is usually a huge output when this happens. He has been worked up for Cushing’s and diabetes mellitus and has had two ultrasounds of his abdomen. None of these evaluations have come up with a diagnosis. His Alk Phos has been in the 500 range, increased from mid 300’s two+ years prior, but that has remained stable during the past nine months. I am wondering if the Vetmedin is acting as a diuretic and am tempted to take him off it at least for a while to see if it makes a difference. Is there any negative aspect to doing this as a trial? Your opinion would be welcomed. Thanks.
Hi Kate. There are a few issues here. Generally, we don’t put animals on pimobendan solely due to the presence of a heart murmur when other diagnostic tools are available. Therefore, it’s possible that your dog does not need it, and that radiography or ultrasonography would help answer the question. As you can imagine, these tests can be quite cost-effective given the cost of the medication itself. It’s also possible though, that your vets are quite certain of the severity of the heart disease, which would indicate a high-grade murmur. Regarding the possibility of a diuretic action, I am unaware of this and have not seen it in practice. A trial off the medication would be hazardous if it was assisting cardiac function, which is hard to judge as the heart could have worsened since the medication started, with this worsening masked by the positive drug effect. I assume you are quite certain that you’re not also giving a diuretic, either separately or combined, as these are very commonly used in heart disease.
Our dog also has problems with incontinence and excessive drinking of water since starting cardisure. Also some vomiting.
Hi Sherrie. Is he or she also on frusemide or a similar diuretic? I’m only asking because increased water intake and incontinence are not typical side-effects of pimobendan. It also makes me wonder about underlying kidney function so hopefully there have been recent blood tests.
I have an 11 yo Doberman who was diagnosed with DCM in March of 2022 he was put on Furosemide 1 tab twice a day (up to 4x a day), Enalapril 3 tabs twice a day and Pimobendan 1 tab twice a day. He had been doing great but every so often has a hard time breathing. If it’s a raspy breathing we give him another dose of the Furosemide. If it’s just hard breathing we give him 2 tabs of the enalapril. Sometimes the third one. I don’t want to overdose him but want him to be comfortable. More times than not he is going great and still has puppy in him. We haven’t done any extra of the Pimobendan but wonder if that would maybe help if needed. Would it be better to give him an extra dose of the Pimobendan rather than the enalapril?
Hi Susan. Although I can’t answer your question directly, it is fair to say that enalapril and furosemide are typically more toxic when given in excess than pimobendan. I certainly have given higher doses than you are currently using to some dogs (depending on the weight, of course) and it’s a question you can bring up with your vet.
Hi, my 12 year old chihuahua was diagnose with a heart murmur recently , the vet prescribed Enapril and Pimobendan plus Frusemide diuretics, she had horrible side effects i thought she was going to die in front of me, not eating, dizzy, chills trembling not able to walk well, i call the vet to ask him if i can take her out of these meds the vet said to give a low dose but it was the same side effects she lost a lot of weight in 2 weeks i think the vet prescribed a lot meds for a 5 pound chihuhahua, she is now without the meds and she is doing fine, those drugs were to much for her, I hated to see her miserable, rather see her well until her time is due hopefully little bit longer.
Hi Estela. That’s a difficult one to unpack, and I’m not sure I can comment. With so many drugs at the same time (which is not incorrect to do) it’s very hard to decide which, if any of them is the problem, or if it’s the underlying disease failing to respond to treatment and instead getting worse.
My lovely dog Mia (cavoodle, nearly 9 years old) has a murmur due to a faulty heart valve. She started on Cardisure 2.5 mg tablets twice daily 6 weeks ago.
Won’t pimobendan increase pressure (or force) on her faulty valve and cause it to deteriorate faster? I know she needs pimobendan to improve heart failure (causing coughing & panting), but could she have an earlier death (eg, if the valve deteriorates faster due to the increased cardiac output)? I know what the clinical trials have shown but I still have concerns.
After 6 weeks treatment, she has already deteriorated significantly and now pants heavily for hours after a short walk. It’s so sad because she loves going for long walks, but her walking days are over. We’ll buy her a trolley so she can still be with us. We love her so much!
Hi Mark. The answer your question comes down to whether you are prepared to go with the evidence or not. If the end goal is improved lifespan and quality of life, it should be straightforward to go out on a limb and use this drug given what we know. Always question whether it’s the drug, or the disease that is causing the signs, and whether in fact what is needed is a more tailored treatment, rather than less of it.
My 16 year old cocker x poodle is on Cardisure for heart, Inflacam for arthritis and paracetamol for general pain. She is deaf, unsteady with muscle wasting on back legs, quite a lot of confusion and seems quite miserable except when she’s eating. She paces around for hours at night. Do you think I should stop the medication… and let her go naturally. She loves her food and has no problem going to the loo. She recently had a bad UTI and is on antibiotics. The infection seems to be improved but she is drinking a lot…I suspect this is going to be a diagnosed kidney problem. I don’t want her to live being so miserable but I also would like her to pass peacefully without euthanasia, if possible.
Hi Candy. That’s a decision that can only be made by people who are intimately acquainted with your dog’s quality of life. Good luck.
My 14 year old chihuahua/terrier cross has been treated with Pimobandan and Frusemide for well over a year now. Yet even though she has a cough that has progressively worsened over, my Vets keep saying to keep things as they are because her lungs sound fairly clear. However, my relative who is a Consultant Doctor has advised me to ask my Vet to put her on some Enalapril as well because this should relieve some fluid from the enlarged heart which is pressing against the bronchi and causing the coughing. His own dogs in the past have been treated with this combination and definitely reduced the coughing.
How do I get my Vets to consider this? Or do I just change my Vets?
Thanks for your advice
Hi Maria. What you suggest is quite reasonable, and at first instance, I would bring it up with your own vets. You might find that they are quite open to it.
My 15 year old teacup Maltese has been on pimo for 6 years. He went into CHF in February of 2016. I believe this has extended his life significantly.
Hi. My 12 yo Chihuahua has then put on pimobendan and I have been giving it to him with food. Now I am seeing on the internet that it is supposed to be given on an empty stomach. When I called my vet they said no, you can give it to him with food don’t worry about it. How much less effective is it if given with food? My little guy is terrible about taking pills.
Hi Jennifer. I don’t believe that the medication has been trialed with food so it would be hard to say. What we advise people is to try on an empty stomach, but if you can’t then give as little food as possible and wait an hour to give a full meal.
Even if your vet said it’s fine with food, as long as the official prospect says it should be given on empty stomach/one hour before meal, following the prospect would insure maximum absorption and efficiency. The way you give the pills to him and ensure minimal food in stomach at that time, is to wrap them into some soft dog treat, e.g. Royal Canin Pill Assist (S size) or Dechra Specific Organic Treats – since the pills are small you can use half or a quarter of the treat to wrap it around.
My 10 yo ex-puppy farm toy poodle is on Cardisure but only half of the 1.25mg tablet daily in the early morning. He was originally also taking Frusemide but only for a short course. I also seem to have Fortekor Plus in my arsenal but can’t remember why! His cough has re-emerged and he is not due for his re-assessment for another almost 2 weeks. I seem to recall he didn’t respond as well as anticipated to the full dose of Cardisure so our vet halved the dose. Should I reintroduce the Frusemide in these next two weeks? He’s such a healthy happy little fellow otherwise.
Sorry. You’ll need a vet that can physically examine your pup to answer that. Though I wish you could use the full dose twice a day.
Dr Andrew, I have a 15 yr Chihuahua with a bad heart murmur Vet has heard water on her lungs for 6 months she makes all kinds noise while sleeping she’s had about 6 seizures, over 2 yrs she falls over, stiff neck tilted back crying hearing aids and defecates last night she had another one but after she came out of it she was exhausted, and just slept in my arms all night. She’s was on Pimobendan & Frusemide and added Enalapril Meleate few months ago, 2 weeks ago he changed the Pimobendan to Vetmedin they told me they couldn’t get Pimobendan anymore. She was doing just fine no seizures I plan on discussing this issue with her Vet but I’d would like to know what you think could it be from changing that medication I have read good and bad things about Vetmedin.
Hi Jodinna. Try to avoid reading too much into user reviews about these drugs – here we are dealing with dogs with severe disease, and people often mistake the illness for side-effects of the drug. There should be no practical difference between pimobendan and Vetmedin in any developed country with good quality control regulations as they are the same active ingredient.
Hi my dog has recently been put on this she is a Staffordshire terrier age 10. The vet diagnosed her with a heart mumur after taking her in for breathing issue and after listing to her chest said there was a mumur ( I thought more of a test would of been done to diagnose a dog with heart failure? ) anyway shes on 10mg a day and after eating her pm on she manged to Rob another half . So 24hrs shes had 15mg is she going to be OK?
Hi Hannah. It’s a high dose but she should be fine if it’s not repeated. As for whether you need another test, ask your vet. There may be enough evidence already from the clinical picture.
Dosing with Pimobendan wouldn’t result in a significantly QUIETER heart murmur would it? Nothing in what I’ve read suggests it would.
I’m asking because I can’t see how an elderly dog progressing steadily through the the stages of heart murmurs from grade 2 three years ago to grade 4 six weeks ago should suddenly “improve” to a grade 2 heart murmur.
It’s a particularly dubious “improvement” to my mind because the dog’s heart thumping away was one of the reasons why her foster-carer booked that appointment with an out of area vet 2 weeks ago.
The dog died 2 days ago, almost certainly from heart failure.
Hi Linda. I have a couple of thoughts. Firstly, it is possible that a heart murmur could improve on medication, though it would be rare. Another reason why a murmur could decrease in grade would in fact be that the defect has grown in size. Paradoxical as it may seem, as a hole or gap in a valve gets larger, the noise produced can reduce, much in the same way that a whistle makes noise but a pipe doesn’t. It’s always important to remember that the noise of a heart murmur is not ever an objective measurement but a subjective one and will vary between observers, and also between animals with the same severity of heart disease.
Hello. My 11 1/2 year old Australian Cattle Dog was just started on Pimobendin 2 days ago. At the age of 6 it was discovered he had a stage 3 Pulmonic murmur , but no symptoms, still very active, and did well during his knee surgery. We were told to just monitor. Last fall ( 11 yrs old) we were told the murmur was at a stage 5/6 Pulmonic, AV 2 missing/throwing beats. But he still was very active and no symptoms. Last week I began hearing a few quiet, congested like coughs in early morning sleep, and some heavier breathing. Also he has lost a lot of muscle mass, but thought possibly age related? So I scheduled an appointment with vet, and he said he heard some congestion in lungs, not a lot yet, and that the murmur sounded the same, But he wanted to start this med. I asked about lasix for the edema in lungs, but he said the pimobendin would be better??
Hi Mona. Your vet sounds correct. Lasix (frusemide) may relieve the symptoms, but probably not extend lifespan whereas pimobendan can do both in the right dog.
My 7 year old terrier has just been to the vet who has said she has a mild heart murmur. Our dog is otherwise very healthy. This is the first time this was detected. She said to come back for a check-up in 6 months. Currently, don’t know if it is a genetic issue or how quickly it may progress. Said she could go on pimobendan as a preventative or at a later stage based on the results of the next check.
We are not sure what to do as there are a lot of side effects.
Hi Rhonda. The best you can do is to follow the advice of your vets and avoid online scaremongering.
All these questions have helped me understand this medication . My 15. 1/2 year old chihuahua. Her heart is on a scale 1to6 is a five .being bad . Heart murmur. She was given 6 month’s to a year to live . But it could happen sooner. She is on this medication . I’m understanding this medication better . Her breathing sounds better . Thank you for all the questions you have answered .
My Yorkie turned 12 August 10th of this year. He was misdiagnosed with tracheal collapse and a Cardiologist echo diagnosis is mild B2 , Murmur and left mvd not in CHF and is asymptomatic. Is there better evidence to do nothing at this point or to use vetmedin to reduce enlargement and prolong the asymptomatic stage? I read that another yorkie, younger at nine, was in the same situation but seemed to go into CHF and perish within a couple of months of starting vetmedin. Is it possible, for the valve to burst input on Vetmedin too soon?
Hi Sharon. The evidence presented here is all there is, but it should be sufficient for you to feel comfortable using the drug to prolong the asymptomatic phase. To do anything else is to ignore the evidence and will probably be to the detriment of your dog. Science is the antidote to being overly influenced by single instances like the one you describe.
I desperately need advice for my 16 year old chiweenie. He’s had a heart murmur for years, but recently increased in severity past year…now #4. He collapsed recently, and we rushed him to the vet. He didn’t appear to have any fluid in lungs, but they thought they saw something possibly starting in his lungs. Since there’s been a rash of canine pneumonia, they gave antibiotics along with a diuretic and a prescription for vetmedin. On the four days I had him on the vetmedin, he started breathing heavy and fast…120 breaths a minute, and his heart was very loud…I could hear it beat from the other end of the couch. I called them, they said keep him on it to get used to it. Third day he vomited, erratic heart beat and wouldn’t eat, and collapsed again. I called them and they agreed to take him off the med. He has returned to normal breathing, and started eating again, but he just collapsed again tonight. I don’t know what to do. Is it possible he has something else wrong, or are there dogs that can’t take this drug…and is there any other option?
I have depleted my $ account & care credit is maxed out.
He seemed to be better the past week, but now this. I feel so helpless.
Hi Alyce. It’s impossible to offer advice with such a complex case without being intimately involved. Only your vets can help. Be careful however to separate (as other people are finding in the comments here) the side-effects of the drug from the effects of the disease itself. Your dog was already severely affected before the medication was started, so it’s probably more likely that the adverse effects you are seeing are because the disease is so hard to treat, not because the drug is to blame. It is generally very well tolerated despite what you read online. However, a dog with severe cardiac disease will need a lot more than this drug to control the symptoms regardless.
My 13-14 year old rescued Pomeranian will only take Pimobentan with food. I have to crush the tablet and mix it into his meal. I’m concerned whether he is getting the full complement of the medication. There is no other way for me to give him another form of the drug. He is also on Rimadyl, Tussigon and Gabapentin which are all administered the same way.
Hi Richard. When this happens we advise that you just use as little food as you can. It’s very hard to know how much the food will reduce the efficacy but the drug still seems to work fine if you don’t give a full meal.
Hi my saluki is 11years old and is in heart failure he also has benign tumours on his pancreas
He has been taking Cardisure 10mgs for around 16 months and no other medication my question is when do I know it’s coming to the end of his life as he cannot walk any distance he is extremely lethargic but still has a good appetite
Sorry maybe this is impossible to answer but I’m at a loss to know what’s best for him and vets have not given me any guidance
Hi Denice. You are right that I can’t make specific comments without seeing him, but I can make a few general statements. Firstly, pimobendan used alone is only the beginning of treatment of heart disease in dogs. If it’s his heart disease that is causing his signs, there are many other drugs that can be added to improve his quality of life. If however it’s something else, like arthritis, then that can also be treated very well and I would be asking your vet what can be done to improve his mobility. He is only 11 after all. If other options have been explored without success, then you might start wondering whether if it’s getting close to his time.
My mini poodle is on vetmedin, hydrocodone homatropine and furosemide. Can one of these cause belly bloatness?
Main suspect vetmedin. How can I find out if swelling caused by liquid or gas?
Hi Michele. If you look at my page on the causes of a swollen belly in dogs, you’ll see that one of the most common causes is ascites caused by congestive cardiac disease. This happens to be the most common condition treated with Vetmedin, so it sounds more like the underlying disease than the drugs (which should in fact be helping). Please get in touch with your vet straight away as it sounds like your dog’s condition is worsening and needs dose adjustments and/or other treatments.
Hi There,
My 11 year Old Springier Spaniel, was diagnosed with a hear murmur, and on Xray, diagnosed with an enlarged heart and put on Pimobendan & Frusemide. She has side effects, no energy, confusion, incontinence and vomiting. Shes been on these tablets for 2 weeks, and the vet said leave her on them. Im so worried they are doing more halm than good, is there an alternative? what would happen if i just stopped giving them to her?
Thank you
Hi Tracey. As you can probably see from the article, the effects of pimobendan in extending lifespan are reasonably unique. Therefore, if there is any way you can get your dog to tolerate it, they would likely live a lot longer. Good luck.
Should I stop this med for severe diarrhea?
Hi Misty. It depends if the diarrhoea is an adverse effect of the drug, the state of your dog’s health, and how important the drug is. Only your vet can decide.
Our 7 year old yorkie has just been diagnosed with a heart murmur and put on pimobenden. She has most of the side effects – loss of appetite, lethargy, loose stool, vomiting. She showed no signs of heart issues. Noe on that medication for about 10 days and all these side effects. Can I just take her off the meds?
Hi Marilyn. A heart murmur alone is insufficient as a reason for starting pimobendan, but I am guessing your vets have seen other changes in your dog that concern them. Therefore, before stopping the drug I would talk to them about why they think your dog should be treated. Normally we look for more evidence, especially signs of heart chamber enlargement (stage B2). If the illnesses began after starting pimobendan, they could well be genuine side-effects of the drug.
We are watching our sons dog while he is away. He is a 9 yr old king charles spaniel He is on this med listed above gor heart failure large heart. Its been 2 mo now. And he has a horrible croup sounding cough. Which is getting worse. Is this more due to the disease or the meds. What do you think? .
Hi J. A worsening cough is almost certainly a sign of the heart disease itself worsening, and suggests that he urgently needs additional medication.